Next-Generation Ocular Implants
PolyActiva’s pipeline of ocular implants with PREZIA™ technology are being developed to offer a safe and effective, fully biodegradable therapy for eye care professionals treating patients with a wide range of ocular diseases.
PROGRAM
PRE-CLINICAL
PHASE I
PHASE II
PHASE III
PA5346
Latanoprost Acid 12-month Sustained Release
Open Angle Glaucoma/Ocular Hypertension
TBD
Undisclosed Sustained Release
Inherited Retinal Disease
Partnership with RareSight Inc.
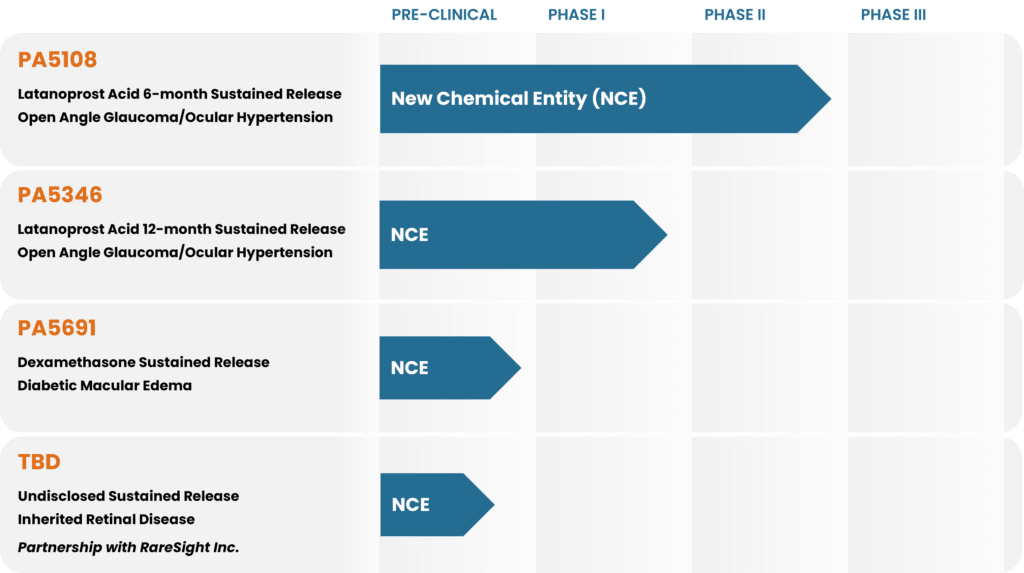
PA5108 - Latanoprost Acid Ocular Implant
Being developed to deliver 6-months sustained dosing of Latanoprost Acid, providing consistent IOP-lowering benefits for patients suffering from Open-Angle Glaucoma & Ocular Hypertension
Current Stage: Phase IIb clinical study ongoing (NCT06964191)
PA5108 has achieved study objectives in two completed clinical studies to date (NCT03604328 and NCT04060758).
PA5108 has not been approved by the U.S. Food and Drug Administration (FDA).
Glaucoma and Ocular Hypertension
Glaucoma is a chronic progressive disease characterized by elevated IOP with progressive optic nerve damage, retinal nerve fiber layer defects and visual field loss. An estimated 3 million adults in the US suffer from Open-Angle Glaucoma and it is the leading cause of blindness. Nearly 50% of Glaucoma patients go undiagnosed since the disease is asymptomatic in the early stages. The damage caused by Glaucoma cannot be reversed, but treatment can slow or prevent vision loss.
Ocular Hypertension is defined as elevated intraocular pressure (IOP) without ocular damage and is associated with an increased risk of progression to Glaucoma. Nearly 7 million adults over the age of 40 in the US are estimated to suffer from Ocular Hypertension.
Micro implant.
Sustained drug release.
The Target Product Profile for PA5108, currently under development, aims to deliver the following clinical benefits:
- 6-month IOP Lowering Efficacy
- Constant Daily Delivered Dose
- Enables Repeat Dosing
- Rapid Biodegradation of the Implant
- Administered in Ophthalmologists' Office
- No Implant Movement
- Minimal to No Endothelial Cell Loss
PA5346 - Latanoprost Acid Ocular Implant
Our second-generation implant, designed to deliver 12-months of sustained dosing in a single administration, allowing for greater flexibility in the treatment regime for reducing IOP in patients suffering from Glaucoma and Ocular Hypertension
Current Stage: Phase I clinical study ongoing (NCT06964061)
PA5346 has not been approved by the U.S. Food and Drug Administration (FDA)
Micro implant.
Extended duration.
The Target Product Profile for PA5346, currently under development, aims to deliver the following clinical benefits:
- 12-month IOP Lowering Efficacy
- Constant Daily Delivered Dose
- Enables Repeat Dosing
- Rapid Biodegradation of the Implant
- Administered in Ophthalmologists' Office
- No Implant Movement
- Minimal to No Endothelial Cell Loss
PA5691 - Dexamethasone Intravitreal Implant
PolyActiva is currently investigating a micro ocular implant designed to be delivered intravitreally for controlled, sustained release of Dexamethasone for patients suffering from back-of-eye diseases such as Diabetic Macular Edema.
The zero-order release and consistent biodegradation profile of PolyActiva’s PREZIA™ technology could potentially address current issues faced with existing treatments, and improve patient outcomes.
Current Stage: Pre-clinical activities ongoing